China approves emergency use of Sinovac’s COVID-19 vaccine for children aged 3-17
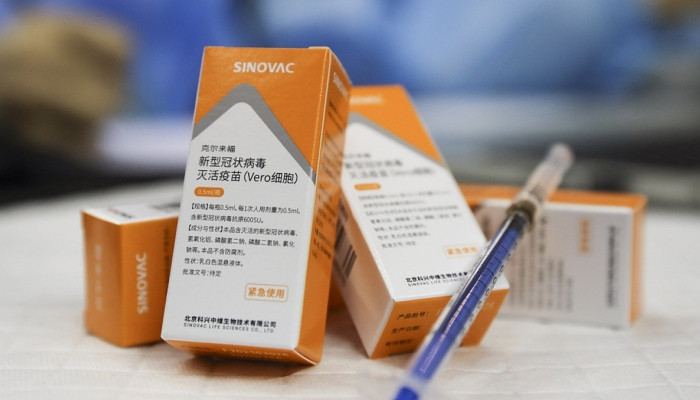 2580 Tuesday, 08 June, 2021, 01:00 China has authorized the emergency use of CoronaVac, the COVID-19 vaccine manufactured by Chinese firm Sinovac, for children aged between 3 and 17, Sinovac Chairman Yin Weidong told the media on Friday. Sinovac confirmed to the Global Times on Friday evening that CoronaVac has been approved for emergency use for this age group. "But when the vaccine will be put into [emergency] use, and starting from what age in the group has yet to be decided," he said. Sinovac has finished the Phrase I and II clinical research stage, involving several hundred volunteers in this age group, which has proved that the vaccine is as safe and efficient as it is for adults, Yin told China Central Television (CCTV) in an interview on Friday. The medicine regulatory authority in Nepal authorized the emergency use of the CoronaVac inactivated COVID-19 vaccine on Friday local time, Nepal-based media outlet The Kathmandu Post reported. It became the 48th country, region or international organization that has approved its use following the World Health Organization (WHO). To date, Sinovac has provided more than 600 million doses of finished and semi-finished vaccine products to nearly 40 countries and regions including China. |

World's Best Hospitals 2026
144209.03.2026, 00:33
Woman who suffered flesh-eating disease after insect bite receives a face transplant
1013504.02.2026, 00:07
India rushes to contain deadly Nipah virus outbreak after five cases confirmed
1356024.01.2026, 20:43
Thousands of NYC nurses strike for better staffing and pay (video)
1487912.01.2026, 23:38
Nestle issues global recall of some baby formula products over toxin fears
1555007.01.2026, 20:43
Air pollution India's biggest health crisis since Covid, warn doctors
1692526.12.2025, 17:41
"What Is This Mushroom?"... Japanese Man Trusts AI, Ends Up in Emergency Room
1871027.11.2025, 22:53
Kenya's refugee camps suffer as US slashes aid (video)
1634411.08.2025, 23:36
